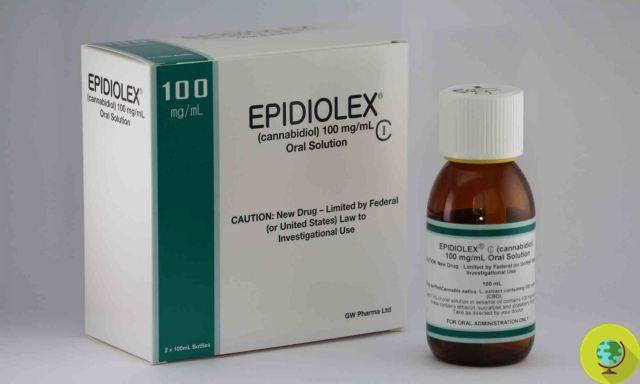
In the United States approved the first cannabis-based drug.
Don't store avocado like this: it's dangerousThe first cannabis-based drug approved by the Food and Drug Administration, the American government body that deals with the regulation of drugs and pharmaceutical products, will treat two forms of epilepsy. It's called Epidiolex, it's a syrup and will be marketed in the States in the fall.
The drug represents the "first pharmaceutical formulation of highly purified plant-based cannabidiol (CBD), a cannabinoid devoid of the high association with marijuana and the first of a new category of antiepileptic drugs".
This means that the drug, produced by a British pharmaceutical company, thanks to the action of cannabidiol will be able to significantly reduce the number of seizures in the case of two specific pathologies: Dravet syndrome (an encephalopathy) and Lennox-Gastaut syndrome (or severe myoclonic epilepsy of childhood), two serious childhood onset pathologies characterized by violent muscle spasms.
As is clear, therefore, the active ingredient of this new medicine is the cannabidiol (CBD), one of the components present in the Cannabis sativa plant. It is not a psychoactive compound such as THC or delta-9-tetrahydrocannabinol, which, on the other hand, is the basis of the pain-relieving and euphoric effects. In contrast, CBD is known in scientific experimentation as an antioxidant and anti-inflammatory, but also effective against schizophrenia, social anxiety disorder and depression.
The medicine acts in reducing the severe seizures that characterize both types of epilepsy (but experts are already looking at other uses) but, although it has proved very effective, the oral solution still has a series of side effects that should not be underestimated, such as diarrhea. increased liver enzymes, weakness, loss of appetite, sleepiness, infections and skin rashes.
In the United States the drug should be marketed already after the summer, while in Europe the European Medical Society - should rule within a few months.
Read also:
- Cannabis light: properties, effects, risks and contraindications of light (and legal) marijuana without thc
- Medical cannabis: where to buy it and everything you need to know
- Medical cannabis does not involve health risks, confirmation from the WHO arrives
Germana Carillo