Laboratori Guidotti of the Menarini group have reported the withdrawal from the market of several batches of their anti-cholesterol supplement with fermented red rice, the LevelipDuo. Also recalled a batch of the Levelip Triol
Don't store avocado like this: it's dangerousSome lots (8 in total) of the anti-cholesterol supplement based on fermented red rice, Levelip duo, and one lot of Levelip triol, were voluntarily recalled by the manufacturer (Laboratori Guidotti), due to "an analytical non-conformity".
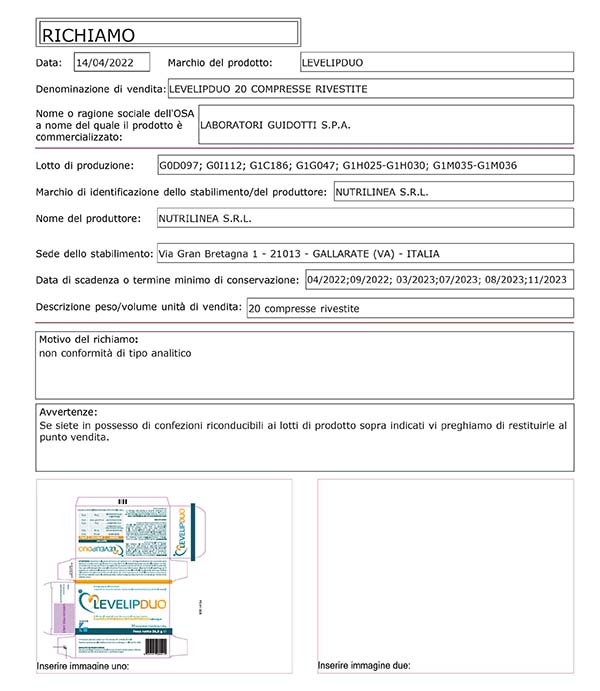
The lots recalled are the following:
LEVELIPDUO 20 COATED TABLETS (Paraf. 978869360 - EAN 8059591080412)
- G0D097 scad. 04/2022
- G0I112 scad. 09/2022
- G1C186 scad. 03/2023
- G1G047 scad. 07/2023
- G1H025 scad. 08/2023
- G1H030 scad. 08/2023
- G1M035 scad. 11/2023
- G1M036 scad. 11/2023
LEVELIP TRIOL 30 COATED TABLETS (Paraf. 979816713 - EAN 8056446340592)
- lotto G0L206 scad. 10/2022

Anyone who has purchased these lots must not consume them but return them immediately to the point of sale.
What is the problem with these supplements?
To reveal in more detail the reason why some batches of these supplements have been recalled is the magazine Il Salvagente which conducted a test on food supplements based on anti-cholesterol fermented red rice.
Among the 15 supplements sampled by the new survey (the complete results of which will be published at the end of the month) there were also those of the Guidotti Laboratories. All products have been tested for disaggregation, a mandatory test only for drugs and not for supplements but which is still very important.
The disaggregation assay, in fact, can verify if the supplement breaks down correctly (within 15-30 minutes depending on the type of tablet) and the body can therefore absorb it effectively. However, it is not certain that, if this does not happen in a precise manner, the active principle will not be absorbed but if there is a non-compliance there is also the risk of a slowed absorption.
According to the Lifebuoy test, the Levelip Duo did not pass the unbundling test and once the result was communicated to the company, it decided to withdraw the batches that presented this problem from the market.
Follow your Telegram | Instagram | Facebook | TikTok | Youtube
Source: CODIFI / Il Salvagente
Read also:
- High Cholesterol: What the Largest Statin Side Effects Study Says?
- High Cholesterol: This "drug-effective chocolate bar" for lowering it
- This is the time you should eat to reduce bad cholesterol, the study