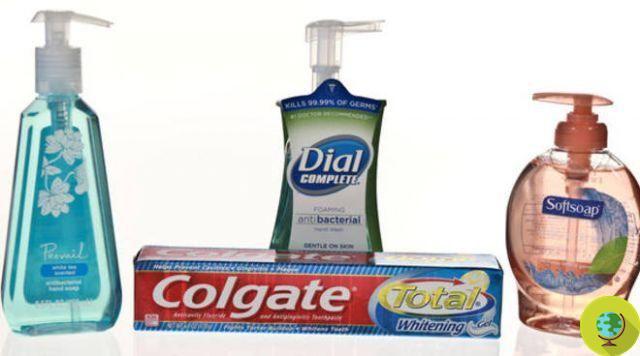
Many people use some products to sanitize their hands that do not need to be rinsed and that are made with different active ingredients of chemical or natural origin. However, hand disinfectants are not always without risks, especially for some categories of people. The American FDA warns and prohibits the use of certain ingredients.
He is about to end up run over, his mother saves him
Many people use some products to sanitize their hands that do not need to be rinsed and that are made with different active ingredients of chemical or natural origin. However, not always i hand sanitizers they are risk-free, especially for some categories of people. The American FDA warns and prohibits the use of certain ingredients.
A new document from the American Food and Drug Administration has established some firm points in recent days regarding the safety and effectiveness of hand sanitizers. The provision, which prohibits the use of certain ingredients in these products, completes a series of actions relating precisely to understanding the safety of commonly used (non-hospital) disinfectants.
The goal is of course to ensure that over-the-counter (OTC) hand sanitizers are safe and effective for those who rely on them and that the ratings on the ingredients they contain are consistent, up-to-date and reflect current scientific knowledge.
The document states that some active ingredients can no longer be used in OTC hand sanitizers, antiseptic products intended for use without water. It is in particular about 28 active ingredients that are not admissible in disinfectants according to the new assessments, among these there is also tea tree oil. The accusation made against these active ingredients, including essential oil, is that of being harmful to pregnant women and children.
The need for further data on three other active ingredients was also reiterated, benzalkonium chloride, ethyl alcohol and isopropyl alcohol, ingredients commonly used in hand sanitizers that we now intend to understand if they are really safe and effective. At this time, the FDA has no plans to take action to withdraw hand sanitizers that contain these three active ingredients from the market.
Some of these active ingredients had already been banned by the FDA itself in a 2016 provision and at the time additional scientific data had been requested to support the safety and efficacy of the most common active ingredients used in these disinfectant products.
Hand sanitizers are a convenient alternative when it is not possible to wash your hands with soap and water, and millions of Americans use them multiple times every day to reduce the bacterial load on their hands and avoid infection.
The Centers for Disease Control and Prevention (CDC) recall that washing hands with natural soap and running water is one of the most important practices that consumers can take to avoid getting sick and to prevent infections. If soap and water are not available, the CDC recommends using an alcohol-based hand sanitizer containing at least 60% of this active ingredient.
Most of the products use their own ethyl alcohol. For this reason, it is believed that the provision on hand disinfectants, which however will only come into force next year, will affect less than 3% of the market precisely because the most common products use this ingredient.
Read also:
- Goodbye Amuchina®! Ecological alternatives to disinfect
- Homemade hand sanitizer: 5 recipes
- Do-it-yourself hand sanitizer gel, with only 3 ingredients