In the US, Pharmaceutical Inc recalled several batches of hypertension drugs due to the presence of carcinogenic impurities
Don't store avocado like this: it's dangerousA potentially carcinogenic N-nitrosoirbesartan impurity found in some pills used to treat hypertension in America. This is the reason that pushed the pharmaceutical company Lupin Pharmaceutical Inc, based in the United States, to recall several batches of the tablets as a precaution Irbesartan and Irbesartan e Hydrochlorothiazide.
From 8 October 2018 to 30 September 2021, the pharmaceutical company received 4 reports of diseases related to the use of Irbesartan. The drugs in question have been distributed nationwide in the US, to pharmacies and wholesalers.
Irbesartan, sold in packs of 30 or 90 tablets, is prescribed for the treatment of hypertension, diabetic nephropathy in hypertensive patients with type 2 diabetes and in cases of increased serum creatinine and proteinuria. In contrast, Irbesartan and Hydrochlorothiazide - also available in packs of 30 or 90 tablets - are used to treat hypertension in patients who need multiple medications to manage hypertension and for whom monotherapy alone is not sufficient. 'Irbesartan.
Here is the list of drugs with the related lots recalled:
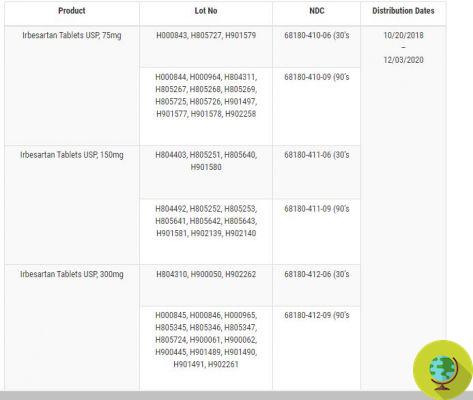
@FDA
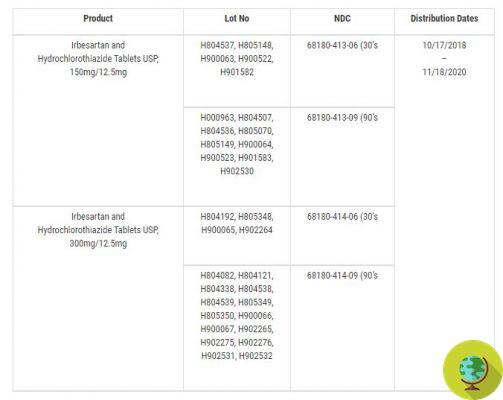
@FDA
As reported by the American Food and Drug Administration, patients who use the drugs object of the recall are required to contact their doctor to continue the therapy with an alternative.
Follow us on Telegram | Instagram | Facebook | TikTok | Youtube
Source: FDA
Read also:
- Aifa withdraws well-known drug against hypertension: check the batches that have impurities
- Medicines and heat: the mistakes you could make in the summer (and that you must avoid)
- 11 batches of diuretic and hypertension drugs withdrawn due to the presence of impurities